
Designation to promote more rapid Agency and market access for VenoStent
HOUSTON, TX / ACCESSWIRE / May 17, 2022 / VenoStent, Inc., a clinical-stage tissue engineering company developing bioabsorbable perivascular wraps to improve outcomes in the 5 million vascular surgeries performed each year, announces that The Center for Devices and Radiological Health (CDRH) at the Food and Drug Administration (FDA) has granted its novel technology, the SelfWrap® Bioabsorbable Perivascular Wrap, Breakthrough Device Designation (BDD).
“We are very honored that the FDA recognizes the promise of VenoStent’s SelfWrap technology to improve the quality and length of life of chronic kidney disease (CKD) patients that require hemodialysis treatments, a life-threatening condition that can be devastating for patients” says VenoStent CEO, Tim Boire. “About a year ago, we embarked on a clinical trial to assess the safety and effectiveness of our novel tissue engineering technology to help CKD patients needing dialysis, and the results of this study are highly encouraging, in terms of both safety and effectiveness. This Breakthrough Device Designation is an acknowledgement from the FDA that VenoStent’s SelfWrap has established ‘a reasonable expectation of technical and clinical success’ with these clinical results and is indeed a promising potential solution for CKD patients. We look forward to all of the benefits that are afforded by this designation, and the potentially profound impact that our device can have on this patient population, as well as others in the future.”

For millions of patients every year, vein grafting offers the best opportunity for survival. These procedures, such as arteriovenous fistula (AVF) creation and coronary artery bypass grafting (CABG), use a vein as a replacement artery. Unfortunately, as veins are not built like arteries, these surgeries can have extremely high failure rates – some more than 50% – greatly increasing morbidity and mortality. VenoStent’s device, SelfWrap, is a macroporous, bioabsorbable polymer wrap that provides scaffolding for these veins, helping them to arterialize and potentially saving thousands of lives in the process.
This BDD is an official recognition from the FDA (“the Agency”) that the SelfWrap technology may provide for more effective treatment of a life-threatening or irreversibly debilitating human disease or condition, with no approved or cleared alternative available to patients. This includes the use of VenoStent’s SelfWrap to improve outcomes in CKD patients that have failed or failing kidneys and are referred for vascular access creation surgery as they require hemodialysis treatments to filter out toxins in their blood that healthy kidneys would normally process. The designation brings with it a more collaborative relationship with the Agency, the potential for more adaptive clinical trial designs that can expedite FDA approval, and more rapid mechanisms for reimbursement to accelerate the availability of innovative, potentially life-saving technologies such as SelfWrap for patients.
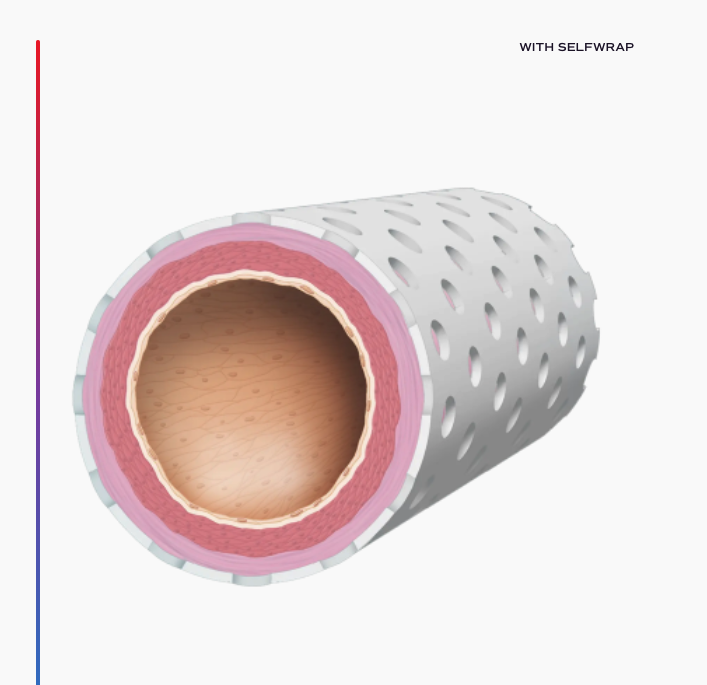

Breakthrough Device Designation
The Breakthrough Devices Program from the FDA was created to assist companies developing promising, novel technologies treating life threatening conditions. The benefits of receiving Breakthrough Device Designation by FDA include improved Agency access with priority review, an accelerated approval process through the potential of more efficient and flexible clinical trial design, and the potential for early reimbursement access through the Transitional Pass-Through Payment and Transitional Add-On Payment programs provided by the Centers for Medicare & Medicaid Services (CMS). Individually, each one of these benefits is impactful for novel medical technologies. In aggregate, they can be transformational for a company like VenoStent, where all of these features of the program can be leveraged to the benefit of a highly vulnerable patient population.
“This is a major step towards helping chronic kidney disease patients in the United States. We are grateful to the team at FDA, our own team, and all of those who have backed us over the years,” says CEO Tim Boire.
About VenoStent
VenoStent is a clinical-stage tissue engineering medical device company developing bioabsorbable smart polymer wraps to transform outcomes in vascular surgery. VenoStent’s initial focus is on extending the quality and length of life of dialysis patients with a polymer wrap that goes around blood vessels to act as a tissue engineering scaffold and prevent failures. This approach can be readily adopted to improve vascular surgery outcomes for patients that suffer from peripheral artery disease (PAD) or coronary artery disease (CAD) and require arterial bypass grafting surgery (peripheral artery bypass grafting, PABG, or coronary artery bypass grafting, CABG), giving this technology the ability to treat over five million patients worldwide.
Forward-Looking Statements
Some of these statements are forward looking and the product discussed is in the investigational stage. It is not available for sale in the US nor worldwide.
Media Contact:
Jennifer Horspool
949-933-4300
[email protected]
SOURCE: VenoStent
View source version on accesswire.com:
https://www.accesswire.com/701726/VenoStent-Technology-Receives-Breakthrough-Device-Designation-by-FDA