![[Vaccine Innovation] Microneedle vaccine patches to the rescue < Special < Article [Vaccine Innovation] Microneedle vaccine patches to the rescue < Special < Article](https://hyperwarped.com/wp-content/uploads/2023/02/20354_20396_424_v150.jpg)
With waning Covid-19 and flu vaccination rates globally, it’s clear that vaccine manufacturers need to get more creative if they don’t want their vaccines left sitting in warehouses instead of in arms.
Some vaccine manufacturers have already started combining seasonal flu vaccines into one syringe. Pfizer began phase 1 trials for a combined Covid-19 and influenza shot to raise vaccine demand again. Soon, RSV vaccines may also be added to that combination shot.
However, other manufacturers are targeting microneedle vaccines both domestically and internationally, and not just for vaccines but a range of other therapeutics. Vaccine manufacturers are investing in this technology to overcome hypodermic needle phobias, injection pain, and cold chain logistics, and improve overall convenience in the vaccine administration process.
What is the technology behind microneedle vaccines?
Transdermal drug delivery systems (TDDS) are not a particularly new concept. Scopolamine patches were first approved for motion sickness in 1979, followed by nitroglycerine patches in 1981, and later gained popularity in nicotine patches for smoking cessation and pain relief patches for athletes. But there are still no approved vaccine patches.
However, the technology inside these and present-day therapeutic patches being developed differ. For example, passive transdermal delivery utilizes natural diffusion to release the drug into the body through the pores of the skin. However, more advanced methods using active transdermal delivery require an agent to help facilitate the transfer via chemical enhancers and permeators, micro-needles, low electrical currents or voltages with iontophoresis and electroporation respectively.
Still, transdermal drug delivery technology is rapidly advancing, and it may not be long before there is one also for vaccines. At least that’s the hope from the Coalition for Epidemic Preparedness Innovations (CEPI).
CEPI announced a $4.3 million investment two weeks ago for a needle-free, high-density microarray patch (HD-MAP) from Vaxxas which is scheduled to undergo preclinical testing to assess its stability, safety and immunogenicity to deliver a rapid-response technology for heat-stable, dried-formulation mRNA vaccines.
Vaxxas’ HD-MAP delivers the vaccine to immune cells directly beneath the surface of the skin via the microneedles. The microprojections also trigger natural immuno-cellular alarms that cause vaccine components to be rapidly directed to lymph nodes, thereby eliciting a robust immune response.
Presently, the cold chain shipping requirements for mRNA vaccines are between -80°C and -60°C for Pfizer and -20°C in Moderna which disproportionately affects low-income countries that cannot facilitate the storage of these vaccines with special refrigerators.
These low temperatures are used to ensure the thermostability of the vaccines as they usually contain ingredients that are unstable at higher temperatures. Microneedle vaccines help overcome the cold chain requirement by delivering the vaccine in a dried formulation.
Testing this technology out at home and abroad
Corium, a U.S.-based biopharmaceutical specializing in CNS healthcare, first obtained approval for the transdermal drug delivery donepezil patch in March 2022. Later, in the Korean market, Icure obtained the Ministry of Food and Drug Safety’s approval for a donepezil patch in November 2021, launched it domestically through Celltrion in August 2022, and is scheduled to enter phase 1 trials in the U.S. this year.
Meanwhile, GC Pharma is currently collaborating with Vaxess Technologies and has already proven strong safety and immunogenicity profiles in the interim results of phase 1 clinical trials of its patch-type influenza vaccine, MMIX-Flu, in December 2022.
The vaccine is characterized by attaching a fine needle that can slowly release the vaccine for a sustained drug release profile without the need for separate refrigeration and distribution requirement, and can reduce the pain at the injection site.
Through funding from the RIGHT Foundation, Abion, and Raphas are currently undergoing preclinical trials for another microneedle technology but this time developed by a local company called Raphas. They employ the method of electroporation which is most efficient at 90 volts but often leaves patients with some pain and skin damage, and thus, Raphas has lowered the voltages to 25 and 50 volts.
Abion and Raphas have already released intermediate results a week ago showing that Abion’s DNA-type vaccine demonstrates strong antibody production at a similar level to that of conventional needle injection DNA delivery methods in pre-clinical trials.
Notably, these trials are expedited compared to conventional clinical trials. Raphas explained that materials with proven efficacy can be mounted on their microneedles. After confirming equivalence to an existing licensed item in phase 1 trials, companies can then move straight to commercialization, skipping phase 2 and 3 clinical trials.
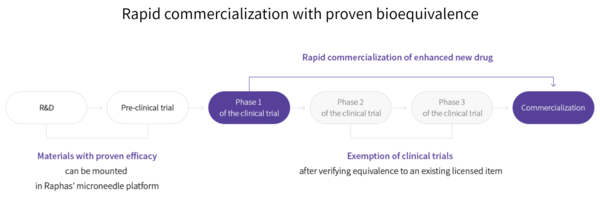
An official at Raphas’ R&D department told Korea Biomedical Review that the company is engaged in projects with another pharmaceutical company besides Abion and a global university but could not disclose the names as yet due to a confidentiality agreement.
Raphas is currently also receiving government funding through a national project to further advance this technology.
“Besides the DNA Covid-19 vaccine, we are also developing many pharmaceuticals with our technology including influenza multivalent vaccines and other chemical drugs,” he added.
Opportunities and factors to consider
The vaccine demand for Covid-19 is currently low but translating vaccines into patches alongside multivalent vaccines could help turn the tide. Aside from the aforementioned benefits, transitioning to this vaccine delivery method also enables smaller doses to be used, meaning less wastage of limited supplies and greater vaccine access.
Also, depending on the specific microneedle technology employed, it can also eliminate the need for a clinician to administer vaccines in the future. CEPI is envisioning a future where microneedle vaccines can be mailed directly to homes, workplaces, and schools, thus easing the vaccine scheduling and administration process.
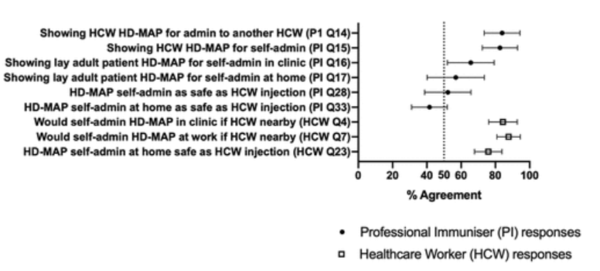
A survey on Vaxass’ HD-MAPs acceptance rate showed that out of 61 participants, the majority showed a high acceptance of the technology ranging from 42 to 83 percent depending on who administered it. Interestingly, a higher acceptance rate was shown if a healthcare worker administered the vaccine or if it was administered in a healthcare setting, thus showing some skepticism towards self-administration at home.
Another factor worth considering is that these technologies can also drive up the cost of vaccines as it is a more advanced technology compared to conventional syringes.
Thus, partnerships with CEPI, other global organizations and the government will be key to get this microneedle technology out into the real world and most importantly, into arms.