Cannabinoid receptors are a popular therapeutic target for cannabinoid-based drugs in the treatment of pain, neurological disorders and inflammation, according to GlobalData’s Pharma Intelligence Centre Drugs database. In 2022, a surge in the pipeline has led to cannabinoid receptors becoming the most popular target in preclinical development. Despite this, the medical use of cannabinoid drugs is heavily restricted, including being banned in 137 countries, according to the United Nations.
Cannabinoid-based drugs are derived from compounds found in the cannabis plant. The two types of cannabinoids targeting cannabinoid receptors are endocannabinoids, which are naturally occurring endogenous ligands and synthetic cannabinoids, which are designed in a laboratory. Cannabinoid receptors fall into two categories: CB1 and CB2 receptors. CB1 receptors are primarily expressed in the brain and central nervous system, whereas CB2 receptors are expressed in T-cells in the immune system.
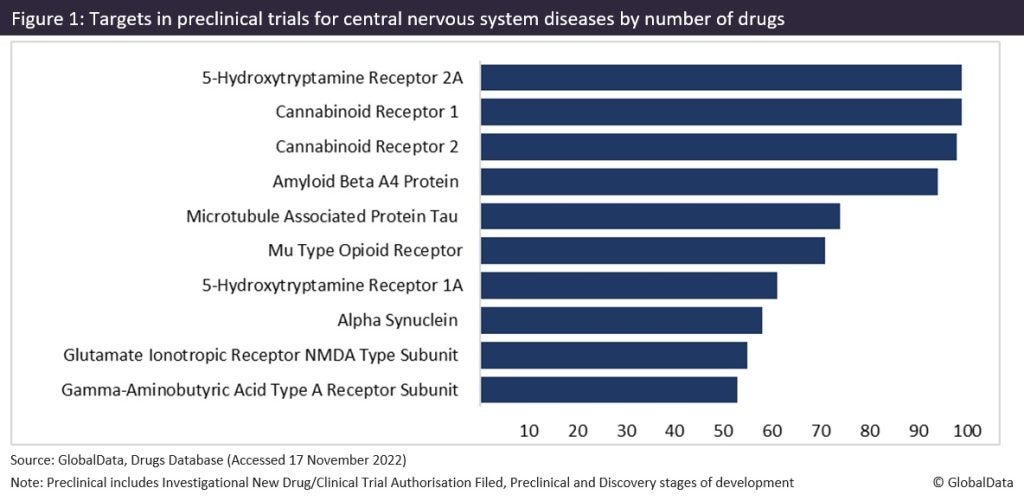
Figure 1 shows the targets in preclinical trials for central nervous system diseases, as sourced from GlobalData’s Drugs Database. CB1 receptors are the joint first most popular target with serotonin receptor 2A in preclinical trials for central nervous system diseases. This is closely followed by CB2 receptors in second place. Collectively, cannabinoid receptors (CB1 and CB2) are currently the most popular targets in preclinical stage of development, with 391 drugs tagged in total.
The most popular indication for cannabinoid receptor drugs is neuropathic and general pain, which accounts for approximately 27% of the drug pipeline. This is followed by epilepsy at 8% of the pipeline and multiple sclerosis at 7% of the pipeline. Several companies are leading in preclinical development, such as Cannabis Science, which has eight drugs in the pipeline with a focus on treating cancers and age-related diseases, and Aphios, which has four drugs in development for treating infectious diseases and cancers.
Despite the current popularity of cannabinoid receptors, the regulatory landscape is challenging and complex. In the US, the world’s largest pharmaceutical market, most states have legalized cannabinoids for medical use. However, at the federal level, cannabinoids remain illegal under the Controlled Substances Act. In most EU and Commonwealth countries, including the UK, New Zealand and Australia, cannabinoids are legal for medical use. However, access is restricted, requiring a special permit given under exceptional circumstances.
In Asia, Africa and the Middle East, cannabinoid-based drugs are mostly illegal and continue to present a major regulatory obstacle. China and Japan have some of the world’s strictest regulations on cannabinoids, including bans on cannabis for medical use and scientific research, as the drug is classified as a dangerous narcotic. In contrast, Latin American countries such as Brazil, Uruguay and Mexico are early adopters of medical cannabis. These countries utilize full legal oversight on cannabinoid medical use, cultivation and trade, which has established a robust regulated market, helping the region to deal with future developments.
Despite the growing popularity of cannabinoid targets, the current regulatory landscape poses a challenge for cannabinoid-based drug developers and regulators. Strict policies in Asia, the Middle East and Africa could lead to restricted progress in late-stage development, which could impact the future market. To ensure the future growth of cannabinoid-based drugs, more progress is required in these regions to ensure advancements in legalisation, oversight and regulatory alignment.