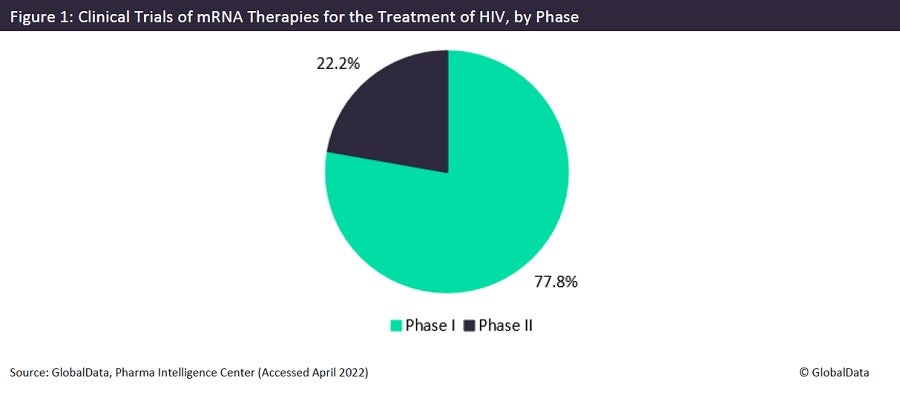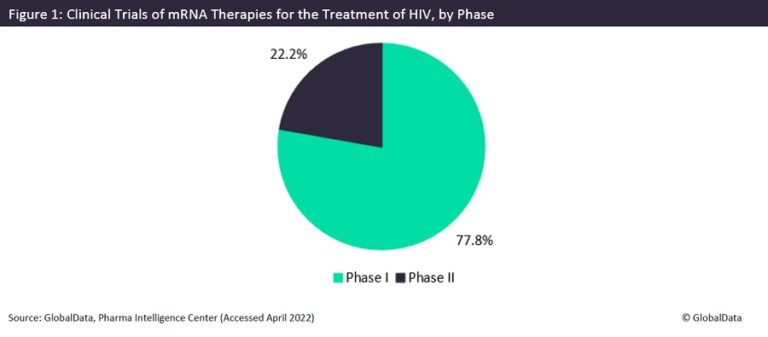
Messenger RNA (mRNA) is a single-stranded RNA molecule that uses one of the DNA strands in a gene to create proteins. It has recently been in the forefront of discussion as the main component in Pfizer’s and Moderna’s approved Covid-19 vaccines. Sponsors are now looking for other uses of mRNA technology, notably in the treatment of human immunodeficiency virus (HIV). There are currently no approved mRNA drugs for the treatment of HIV, but there are nine trials of mRNA-based therapies for the prevention of HIV, as seen in Figure 1. Most of these trials are currently in Phase I (77.8%), while the rest are in Phase II (22.2%).
The most recent trial was initiated on 11 February by the National Institutes of Health (NIH) and the National Institute of Allergy and Infectious Diseases (NIAID). This Phase I clinical trial, called HVTN 302, will test three mRNA candidates for the treatment of HIV: BG505 MD39.3 mRNA, BG505 MD39.3 gp151 mRNA, and BG505 MD39.3 gp151 CD4KO mRNA. The vaccine candidates stimulate the immune system to produce a specific type of antibody against the antigens and help the immune system recognise the antigens in the event of future exposure.
A total of 108 subjects aged between 18 to 55 years are planned to be enrolled into six study groups. Each study group of 18 subjects will take either a low (100mcg) or high (250mcg) dose of one of the three candidates via intramuscular injection to evaluate its safety and immunogenicity. Subjects will be enrolled at sites across the US, including Boston, Los Angeles, New York City and Philadelphia, to name a few locations. The trial is expected to be completed by July next year.